Today, lithium ion batteries power much of what we use: electric cars, laptops and of course, our smartphones. Most people don’t understand how they work or the best practices to extend their life.
In one sense, though, the inner workings of these batteries are pretty simple.
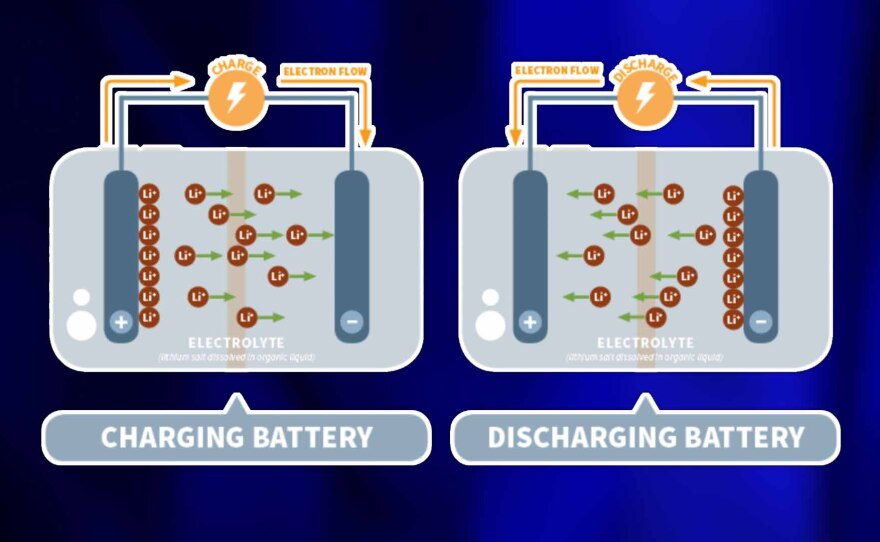
“The operation of a lithium ion battery is really lithium ions moving back and forth … between this positive electrode and this negative electrode,” said Kent Griffith, a chemistry professor at UC San Diego who runs a battery lab.
“And when they move back and forth is when ... you’re able to power your device — when they’re moving from the negative electrode to the positive electrode or when you’re charging your battery. You’re taking them out of the positive and putting them in the negative.”
Griffith told us that movement of ions and electrons inside the battery determined how long your charge would last. And how long the battery itself would survive.
One rule of thumb for protecting the life of the battery: Don’t keep it plugged in and fully charged for a long time.
“When it's 100% charged, that's the worst thing for a battery. Because that’s when degradation happens the fastest,” Griffith said.
A good example of this is a portable computer or laptop that’s constantly plugged in. Its battery is always fully charged, so it’ll wear out really fast.
Another rule: Batteries degrade quickly when they are hot.
“If you can avoid your battery being at 100% state of charge and you can avoid it being hot, say sitting on the dashboard of a car in the sun, that will really improve the longevity of your device,” Griffith said.
Here’s one more tip on how to lengthen your battery’s charge. When you’re traveling long distances, lock onto a moving Wi-Fi signal or put your cell phone on airplane mode.
“If it doesn’t have a strong signal and it’s trying to find one, such as when you are actually flying on an airplane, it’s going to work really hard and drain the battery quickly, and it’s a futile exercise. There’s not going to be a signal to be found from a cell phone tower.”
Griffith’s research focused on trying to find ways for batteries to work better. In his battery lab you would find materials and tools like a “glove box” to assemble materials in a dry, low-oxygen enclosure. Lithium can ignite when exposed to normal air.
He said for now, nothing beats the lithium ion technology at what it does.
“Let’s get one thing sort of straight. Lithium ion is the best technology that we have, right? That’s why it is almost universally used in terms of how much energy it can store. Lithium ion works really well,” Griffith said.
But lithium is a limited resource that has to be mined. One thing Griffith is investigating is the possibility of creating sodium ion batteries. Sodium, he said, is in virtually infinite supply.
“So, if we could make the switch to sodium ion batteries, we wouldn’t ever have to worry about running out of sodium. So there is a sustainability aspect to it and there’s also potentially a cost aspect to it. So lithium is far more expensive than sodium,” he said.
But for now, lithium ion batteries are standard. So let your cell phone or laptop run down and be recharged. And don’t leave it too long in the sun.





