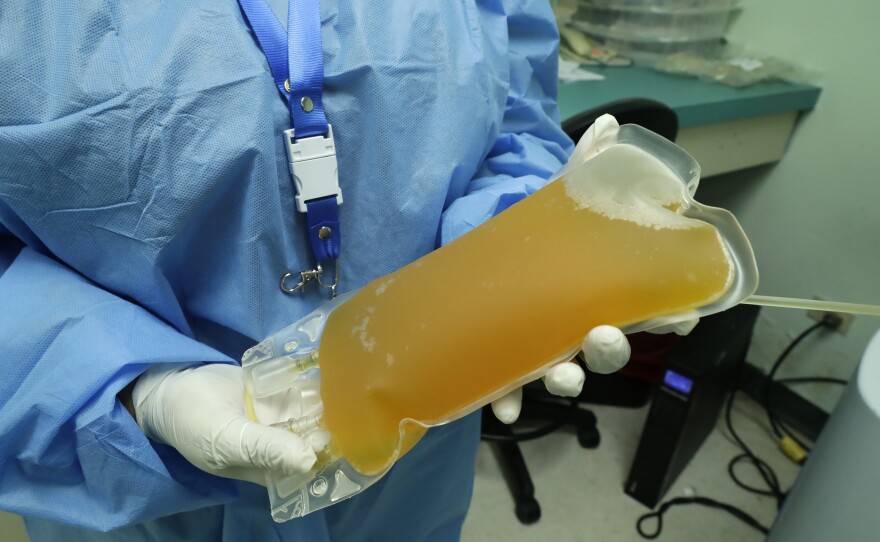
San Diego scientists say the U.S. Food and Drug Administration’s support of convalescent plasma as a “breakthrough” treatment for COVID-19 is unfounded. These scientists say there simply isn’t enough data to prove such a treatment is safe and effective enough for all patients. Now, some scientists are asking the FDA to walk back statements on plasma’s “promising” results.
And that lack of data means it’s still unclear whether convalescent plasma is an effective and safe treatment for all COVID-19 patients. Scientists, who were critical of President Donald Trump’s characterization of plasma as a “breakthrough,” include Dr. Eric Topol of Scripps Research. In an open letter published on Medscape, Topol wrote to Commissioner Stephen Hahn, “You need to organize a press conference and tell the truth.”
“Tell Americans exactly how you were pressured to make a breakthrough announcement. Tell all of us how you completely misrepresented the facts about convalescent plasma, and not hide this with the obscurity of technical terms such as relative and absolute differences … Otherwise, you need to resign.”
Hahn spoke of plasma’s early “promising data” at an Aug. 23 press conference, where the FDA issued emergency use authorization for plasma.
“In the optimal patients as described by Secretary Azar, there was a 35% improvement in survival, which is a significant clinical benefit. Now we’re waiting for more data, but this clearly meets the criteria we’ve established for emergency use authorization. And we are very pleased with these results,” Hahn said at the press conference.
But Topol wrote the data was cherry-picked from “a retrospective, observational study of over 35,000 patients who received convalescent plasma, without any controls or untreated patients for comparison.”
“There was a pre-print that had cherrypicking analysis that raised the hypothesis that there might be some benefit of convalescent plasma, but to call it a breakthrough was preposterous...giving people false hope about a treatment, which we still don't know,” Topol told KPBS.
“We know that the antibodies that people make after infection, most of the antibodies are of no value. Only a tiny fraction are truly neutralizing potent anybody. So to make this declaration and to make this pronouncement of saving 35 lives per 100 who are sick, you know, it's absurd,” he said.
The statement of plasma’s absolute life-saving benefits will make it hard for more clinical trials on plasma to be conducted, Topol said. That’s because clinical trials have to begin with a question of whether or not a treatment will work.
When KPBS reached out to the FDA about these concerns, a spokeswoman only directed KPBS to press releases, which said the emergency use authorization was based on the totality of available scientific data. And they say, the FDA still encourages clinical trials on plasma.
The National Institutes of Health released a statement saying there’s not enough “well-controlled, adequately powered randomized” clinical trial data results to show that plasma is safe and effective enough for COVID -19 patients.
Another scientist, virologist Sumit Chanda of the Sanford Burnham Prebys Medical Discovery Institute, said the conference was disturbing to him as well. Following what he called the FDA”s “premature” support and emergency use authorization of antiviral hydroxychloroquine, which the FDA had to revoke, the absolute support of plasma, with limited data, was too much for him to stay quiet.
“Our entire medical treatment strategy relies on our trust in the FDA. Nobody is looking at the clinical trial data going, ‘hey, should I take this drug or not?’ We trust that the FDA is not using anything but the best science,” he said.
“And that's no longer happening. And I feel that as scientists, we have an obligation to inform the citizenry to say, ‘hey, listen, this is fundamental to our way of life and our health and safety.’"
Chanda said he’s worried a vaccine will be fast-tracked.The CDC has already sent letters to state governors to be ready to distribute a vaccine by Nov. 1, just before election day. But, Chanda says that’s not a safe timeline. Most vaccines are still in clinical trials, and that data has to be reviewed for safety, which could take until at least the end of the year, he said.
“I'm not saying that getting a vaccine out as quickly as possible is not the right thing to do. But as quickly as possible means doing the right types of studies. You're going to do more harm than good if you fast track a vaccine that either isn't efficacious, which is the best case scenario for a vaccine that doesn't work,” he said.
“Giving a drug to somebody who's not sick, that you don't understand what the safety and efficacy profile is, is ethically just just unapproachable.”
CNN reported Tuesday that major drug companies, including Pfizer and Johnson & Johnson, working on a COVID19 vaccine issued a public letter vowing not to bow to political pressure from Trump; that any vaccine will go through rigorous trials necessary to ensure safety and effectiveness. Trump, a day earlier, reiterated his belief that “vaccines will be on the market” before November.