San Diego-based Stemgenex is asking regulators to let patients have access to stem cell treatments it calls "life-altering." But patients currently suing the company claim they were charged thousands of dollars for falsely advertised treatments that didn't improve their health.
In a press release issued last week, the La Jolla company outlined its argument against the U.S. Food and Drug Administration's proposed plans for regulating stem cell treatments. Stemgenex argues the proposed rules would treat a patient's own stem cells as a drug, subjecting stem cell therapies to a lengthy and expensive approval process.
Stemgenex's press release claims the rules, if finalized, "will delay access to stem cell therapies in the United States and cause many Americans who are struggling with degenerative disease to seek treatment outside of the county (sic)."
Jeanne Loring, a stem cell scientist at the Scripps Research Institute, spoke in favor of the proposed rules at an FDA meeting in September. Loring believes these kinds of stem cell clinics need regulation because in her opinion they are "selling something that doesn't work, for a very high price, and taking advantage of people who are desperate."
"They don't want to be regulated by the FDA," Loring said, "because they would have to demonstrate that their therapies actually work."
A Stemgenex spokeswoman did not agree to be interviewed for this story and did not respond to questions submitted via email.
According to the company's website, Stemgenex offers treatments that involve liposuctioning fat out of a patient, isolating and processing stem cells from the fat tissue and then injecting those cells back into the patient. The website lists treatments for Alzheimer's disease, diabetes, multiple sclerosis and a number of other conditions.
UC San Diego stem cell scientist Larry Goldstein told KPBS there's no convincing scientific proof that these kinds of stem cell treatments help people recover from any of those conditions.
"The evidence is just not there," he said. "Medical scientists are working very hard to test therapies properly. But we're not there yet for a great many diseases that these companies claim to have therapies for."
Stemgenex, which describes itself as a "leader in stem cell therapy," has five ongoing studies listed on the National Institutes of Health's online database of clinical trials. None of the trials have posted results proving the treatments to be effective. Stemgenex includes a disclaimer at the bottom of its website that reads, "Stem cell therapy is not FDA approved and is not a cure for any medical condition."
Patients currently pursuing a class-action lawsuit against Stemgenex say the company's treatments were ineffective and misleadingly marketed.
One of the plaintiffs, Selena Moorer of Florida, claims she paid $14,900 and traveled to California to receive a Stemgenex treatment in the hope of battling her lupus. But the lawsuit alleges she "received no significant benefit or effect" from the treatment, and was misled by the company's self-reported statistics regarding patient satisfaction.
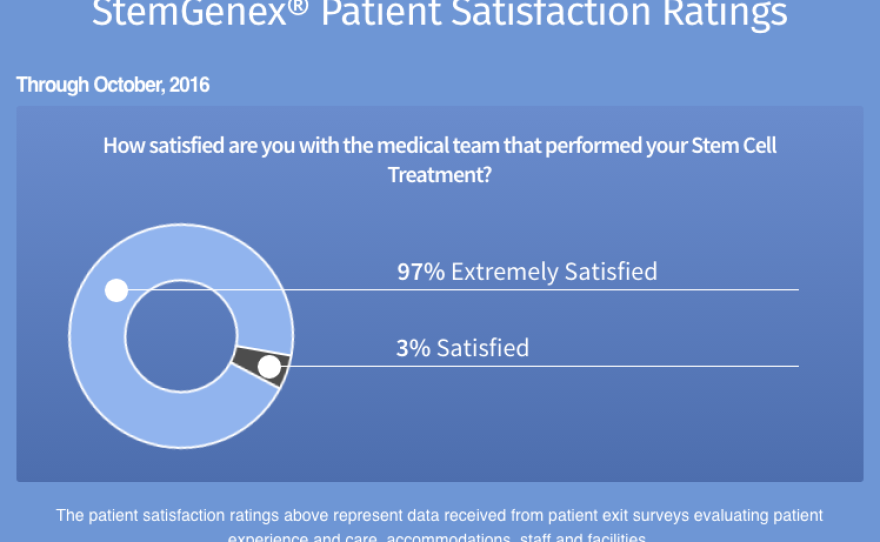
A graph on Stemgenex's website shows a patient satisfaction rate of 100 percent. But the plaintiffs' attorney Jan Mulligan told KPBS, "There can't possibly be the level of satisfaction that they're claiming." She said her firm has been in touch with many dissatisfied Stemgenex patients.
The lawsuit alleges that when Stemgenex was told about Moorer's dissatisfaction, the company offered to sell her a second stem cell treatment at the same price.
The lawsuit also brings forward a complaint of financial elder abuse. Stephen Ginsberg, another plaintiff, allegedly was over the age of 65 when he paid for a Stemgenex treatment and later told the company he "received no effect from the treatment."
The cost of stem cell treatments like those offered by Stemgenex is not covered by insurance or government health plans. The lawsuit alleges that patients who can't personally afford these treatments are "encouraged" by Stemgenex to set up crowdfunding pages on sites like GoFundMe.com.
Outside of the lawsuit, some Stemgenex patients have publicly discussed their satisfaction with the company's treatments. Several spoke positively at the FDA's September meeting. In a video posted to Stemgenex’s Youtube page, one multiple sclerosis patient says she has received two treatments.
"Some people can say it's a placebo effect," she says. "When you're on your feet, you're cooking dinner, and you haven't done that in a year, it's not a placebo effect."
Stemgenex is not the first San Diego-based stem cell company to face complaints from a former patient. As the stem cell treatment industry grows, so does the number of lawsuits filed by patients who say they've been harmed or exploited.
UC Davis stem cell scientist Paul Knoepfler recently co-authored a study showing that hundreds of clinics are currently marketing stem cell treatments throughout the U.S.
"At this time, the vast majority of the clinics seem to be functioning without the oversight that they really need," Knoepfler said. "Part of that could be that clinics are pushing for less oversight."






